Comment;
Interesting research. The caudate nucleus is one of the structures that make up the dorsal striatum, which is a component of the basal ganglia.[1] While the caudate nucleus has long been associated with motor processes due to its role in Parkinson’s disease,[2][clarification needed] it plays important roles in various other nonmotor functions as well, including procedural learning,[3]associative learning[4] and inhibitory control of action, [5] among other functions. The caudate is also one of the brain structures which compose the reward system and functions as part of the cortico–basal ganglia–thalamic loop. Using the MRI Spectroscopy technique to non-invasively assess temperature and chemical changes to look at how well cellular respiration is working is another good approach. The Visual Contrast Sensitivity Test measures mitochondrial dysfunction/impaired oxygen utilization in the rod cells, the black and white receptors in the retina–the “film” of the eyeball (camera). The test is abnormal when the mitochondrial there don’t produce enough energy due to metabolism without oxygen (anaerobic) which is only about 30% as efficient as it normally would be with oxygen. Dr. Younger’s testing shows that this is happening deep in the brain as well.
FEBRUARY 22, 2018
Dr. Jarred Younger (University of Alabama at Birmingham) recently provided Solve ME/CFS Initiative (SMCI) with a progress update on the Ramsay 2016 Research Team 1 project. Dr. Younger is using a magnetic resonance spectroscopic thermometry (MRSt) technique to assess absolute temperature across the entire brain. He aims to describe inflammatory processes at work in the brains of individuals with ME/CFS. In the following interview, he reflects on what motivated him to study neuroinflammation in ME/CFS and how pilot grants like the Ramsay Award can jumpstart promising lines of investigation.

Research update from Dr. Younger
“We are testing the hypothesis that ME/CFS fatigue is due to inflammation of the brain. To test that hypothesis, we are using a type of MRI scan called magnetic resonance spectroscopic imaging (MRSI). This technique allows us to collect 3D, full-brain pictures of several markers that are elevated with brain inflammation. We can measure myo-inositol and choline, which are increased when brain immune cells are activated and lactate which is increased during more severe brain inflammation. The picture below shows an example of an ME/CFS participant, where we can see possible inflammation near the front part of the brain.
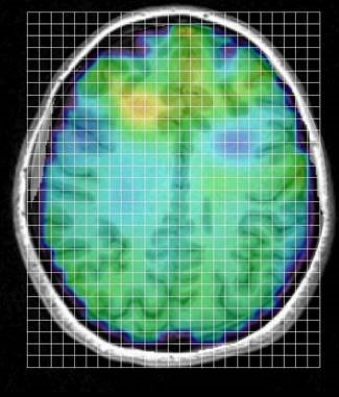
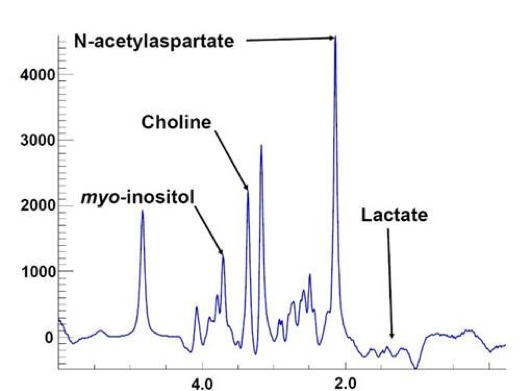
In every single square (or voxel), we get a plot like the one below, that allows us to calculate the concentration of the inflammation markers. We get over 4000 of these plots, set up as a grid, over the entire brain.
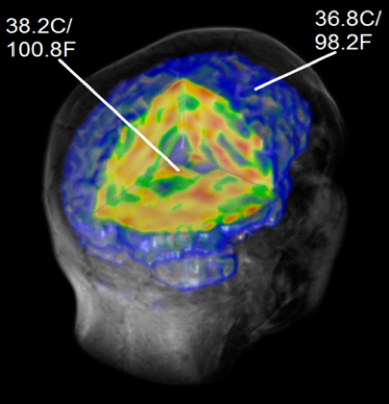
This technique also allows us to calculate true temperature across the entire brain. We believe brain temperature can be a marker for inflammation, just like how a fever is a sign of infection or inflammation in the body. Below is an example participant where we can see that the brain is cooler near the outside and gets warmer closer to the center of the brain. In ME/CFS, we predict the brain temperature will be greater than in healthy controls because the increased inflammation generates more heat.
While we are still collecting data, we are already seeing some interesting results. We have to be cautious in discussing what we are finding, because the story can change as we run more people. But as of right now, we see that individuals with ME/CFS appear to have elevated myo-inositol and maybe lactate across much of their brain, which indicates inflammatory processes. We are also seeing elevated brain temperature deep in the brain of ME/CFS participants, in areas where myo-inositol is increased. One of these regions is the caudate, which could have important connections to fatigue. It is important to note that we have not seen any signs of neuronal damage. There is a marker of neuron health called N-acetylaspartate that was the same between ME/CFS and healthy controls. This is potentially good news because it suggests there is no neurodegeneration occurring.
Q&A with Dr. Younger
- What was a defining moment in your past research and how is it applicable to your current study of neuroinflammation in ME/CFS?
The first ME/CFS study I conducted was in 2011. It was funded by a seed grant from Stanford’s Institute of Immunity, Transplantation and Infection, that was designed to bring new investigators to ME/CFS research. I wanted to know if there was an association between blood-based inflammatory markers and day-to-day changes in fatigue severity. Even though I hypothesized an association, I was still surprised at the results that showed blood markers closely tracked with fatigue. The results from that study were critical to my research career because they pointed me in the direction of looking at inflammation and neuroinflammation in ME/CFS. That pilot grant has since turned into a large, 5-year NIH grant that is a major part of my work. It would have been very hard for me to break into the area of ME/CFS research if that seed grant wasn’t available. So, my own career in ME/CFS research shows that pilot grants are critical to the research enterprise.
- How might magnetic resonance spectroscopic thermometry (MRSt) provide mechanistic insight into the disease?
MRS is a really impressive imaging tool because it allows us to measure brain chemistry in a completely non-invasive fashion. There are no inserted probes, and we don’t have to inject the participants with anything. And we can collect all the data in 20 minutes. We use this technique to get many indicators of ME/CFS pathophysiology simultaneously. One of those indicators is brain temperature. People routinely use body temperature to test if there is infection or inflammation, and we think the same principle can apply to the brain. Inflammation requires more metabolic expenditures, which increases heat that can build up in the brain. So, if we see high brain temperature, we may conclude that there are inflammatory processes at work. We also do a separate scan of blood flow to make sure elevated temperatures aren’t due to inadequate blood circulation. If we can identify neuroinflammation as a factor in many cases of ME/CFS, it will open up new possibilities for treatment.
- You are probing five hypotheses related to brain temperature as a proxy measure for neuroinflammation in ME/CFS, including a biological gradient with disease severity. How would evidence for an association between severity and increased brain temperature strengthen the case for MRSt as a diagnostic method?
While it isn’t essential, it is better when the biomarker correlates with symptom severity. It is just more convincing if a person with mild ME/CFS has slightly elevated brain temperature, while a person with severe ME/CFS has significantly elevated temperature. An association would also make it possible to use temperature as an outcome in clinical trials – we would expect an effective treatment to lower brain temperature.
- What steps did you take to gather a well characterized patient study group and why is this important in the experimental process?
Proper characterization of the ME/CFS group is critical to the success of the project. Fatigue is the most general symptom humans have, and there are dozens of medical conditions that can cause someone to feel profoundly fatigued. It is therefore critical to rule out conditions such as diabetes, adrenal insufficiency, hypothyroidism, anemia, and acute infections that can be mistaken for ME/CFS. We conduct a large number of blood tests on our participants to rule out as many alternative reasons for fatigue as possible. Most individuals with fatigue do not make it through our screening process, and we frequently uncover medical conditions that the patient was not aware they had.
- How has the ME/CFS space evolved generally, and what do you see for its future?
From my vantage point, things are moving in the right direction, just not as quickly as we would like. ME/CFS is increasingly becoming an “accepted” area of research, and more researchers are being attracted to the area. There is a stronger advocacy community, more money for research, and increased attention from the CDC and NIH. There are also more companies trying to be the first to get an FDA-approved treatment to market. All of these developments mean a more tenable environment for researchers to dedicate their careers to ME/CFS (which would have been extremely risky a few years ago). What I am also seeing is findings from a variety of laboratories converging on common stories. The increasing agreement that there are likely at least 3 important ME/CFS subgroups is an important development, as we look at the condition in increasingly sophisticated ways. Funders, government agencies, and researchers are now asking serious questions about how we make sure we are running studies in the best way possible, which improves the quality of the science. We have reached the stage where critical discoveries are now possible.
I believe what will happen in the next few years is that a group will produce an objective and replicable diagnostic tool for a large subgroup of ME/CFS patients. Once that first subgroup is defined and verified, we can more quickly start classifying the remaining patients into subgroups. Those subgroups will be defined by pathophysiology, meaning that a treatment direction will be clearly indicated. I would be excited if we could classify at least 60% of ME/CFS patients in the next five years. I know that sounds slow, but I really think the best way to end ME/CFS is to methodically define each subgroup. And once the subgroups are clearly defined, effective treatments will come much more quickly.
- Has the SMCI Ramsay Award funding been a helpful investment in your work?
Absolutely. This pilot study would not have been conducted without the Ramsay award. The importance of these awards is that they fund early ideas that have the potential to change science and medicine. It is very difficult to get these first studies funded by the National Institutes of Health (NIH) and other large federal agencies. But being able to collect compelling preliminary data from the Ramsay award puts us in a much better position to get a large NIH grant. We submitted a grant application to NIH in February of this year, and the Ramsay award data was the centerpiece of that application. So, I see these awards as critical to jump starting new lines of research.
- COVID UPDATE: What is the truth? - 2022-11-08
- Pathologist Speaks Out About COVID Jab Effects - 2022-07-04
- A Massive Spike in Disability is Most Likely Due to a Wave of Vaccine Injuries - 2022-06-30


