https://www.cnn.com/2018/11/06/health/naloxone-expiration-date-potency-study/index.html
Comment; Clearly we can save money and need more research on long-term stability of drugs, the process must be streamlined with the FDA too.
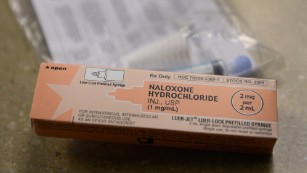
(CNN)Two common naloxone products, Narcan and Evzio, remain chemically stable after their expiration dates, according to new research.
Naloxone, the generic name for the drug, is sometimes called a “save shot” or a “rescue shot” because of its ability to bring someone back from an opioid overdose. It has long been used in hospitals and by emergency medical technicians, but as the opioid addiction crisis sweeps the nation, it has become more widely available to people addicted to drugs and those around them.
Narcan, a naloxone nasal spray, was found to be chemically stable for 10 months after its labeled expiration date. Evzio, a naloxone injection, was chemically stable for at least one year after its expiration date. The products tested were not kept in ideal storage conditions, the researchers say.
“The branded prescription products, the auto injectors and nasal spray, both of them still tested active. Every time we’ve tested them, they are still testing 100% active,” said Charles Babcock, assistant clinical professor in the School of Pharmacy at Marshall University.
Babcock said the results would be expected for naloxone stored in a controlled environment but not for products that had been stored by patients.
“Every one of them tested the same, which is what was impressive, especially considering the fact that we had actually dispensed these products to patients,” said Babcock, principal investigator of the research, presented Tuesday at the PharmSci 360 Annual Meeting.
“I dispense naloxone to patients all the time, and I had a couple come in after it had expired, and they had used it, and people were brought back to life.”
Chris McCurdy, 2018 president of the American Association of Pharmaceutical Scientists, said “this research, in particular with naloxone, is obviously an important one for this day and time because of the opioid crisis that we are facing.” McCurdy was not involved with the research.
The National Institute on Drug Abuse credits naloxone administered by non-medical professionals as saving at least 26,500 people from drug overdoses between 1996 and 2014.
Research like this could lead to changes in the expiration dates of medications, said McCurdy, who is also a professor of medicinal chemistry at the University of Florida College of Pharmacy. More research is needed for regulatory bodies “so we can get meaningful expiration dates on to products that the people can really trust,” he said.
“Showing that these drugs are actually stable beyond their expiration date indicates that we need to go back to the manufacturers and regulatory agencies and get adequate expiration dates on these products.”
However, he does not recommend that people take medication that has expired.
“We go by the expiration date, and we don’t recommend people to take medications beyond their expiration date because, quite frankly, we don’t know if it has decomposed or not,” he said.
Research around drug expiration date testing is beneficial from a health care and an economic standpoint, according to Lee Cantrell, a professor of pharmacy and medicine at the University of California, San Diego who was not involved with the new research. For some medications, extended expiration dates could increase their availability when needed.
“If there were studies that supported that medications remained viable for much longer and medications didn’t need to be turned over so quickly and repurchased, that would be a huge cost savings to the health care system,” said Cantrell, who is also director of the California Poison Control System, San Diego division.
Research on drug expiration dates
Naloxone isn’t the only drug that’s been found to retain its potency after its marketed expiration date.
“I just don’t think we are where we need to be with respect to prescription drug date expiration dating within the United States,” Cantrell said. “This is just additional science that supports the fact that different types of medication maintain their potency for years to decades further out from their expiration dates.”
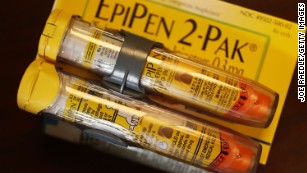
Last year, Cantrell found that epinephrine, the active ingredient in EpiPens, was still present up to 50 months after the stated expiration date.
His sample consisted of 40 expired EpiPens, nine of them EpiPen Jr; the medications are used to treat allergic emergencies. EpiPen Jr contains the correct dose of epinephrine for a patient weighing between 33 and 66 pounds.
The study found that 61% of the EpiPens and 56% of the EpiPen Jrs contained at least 90% of their stated amount of epinephrine.
“They were up to four years expired, and we analyzed them, and we found that they all had a residual dose in them that would be found therapeutic,” he said.
This year, the FDA extended the expiration dates of certain lots of EpiPens by four months to help combat shortages of the products.
Cantrell’s research has found that a number of active ingredients in prescription medication stayed potent after their labeled expiration date. Some of the tested drugs had expired as long as 40 years before.
“Acetaminophen, caffeine, codeine, those are ones that people would probably recognize,” Cantrell said.
Get CNN Health’s weekly newsletter
Sign up here to get The Results Are In with Dr. Sanjay Gupta every Tuesday from the CNN Health team.
Acetaminophen is present in many over-the-counter products to relieve pain and fever and to help treat coughs, colds, flu and sleeplessness. Codeine is used in some cough and cold medicines and as a pain reliever.
Other ingredients that stayed potent after their expiration date included butalbital, methaqualone, phenacetin, meprobamate, pentobarbital, secobarbital, hydrocodone, homatropine and chlorpheniramine.
Although expired medication may still work in some cases, patients are still advised not to take it. The medication might not be as strong as it needs to be to help.
“The best thing is not to take any medication that is past its expiration date and really consult a pharmacist to determine what you should do with that medication to properly dispose of it,” said McCurdy, of the American Association of Pharmaceutical Scientists.
Latest posts by Dr. Raymond Oenbrink (see all)
- COVID UPDATE: What is the truth? - 2022-11-08
- Pathologist Speaks Out About COVID Jab Effects - 2022-07-04
- A Massive Spike in Disability is Most Likely Due to a Wave of Vaccine Injuries - 2022-06-30